Hi there!! You have read the title of my blog post about cinnamic aldehyde and may be thinking of how to define what does cinnamon smells like. So, the answer is that cinnamon has a hot, sweet, tempting, and earthy aroma. Although this spice has a very delicate smell, many of you find it to have a strong odor.
What is Cinnamic Aldehyde

Also known as trans-cinnamic acid, cinnam aldehyde, cinnamaldehyde, or cinnamon is an aromatic compound isolates mainly from Cinnamomum Cassia. It is a naturally occuring flavonoid that gives spice cinnamon a characteristic smell and taste. Are you curious about what cinnamic aldehyde is found in? The cinnamaldehyde is in the woody structures of this genus, such as cassia and camphor.
Don’t forget the past.
Jean-Baptiste Dumas and Eugene-Melchior Péligot isolated this compound (actually trans-cinnamaldehyde) for the first time in 1834 from cinnamon essential oils. In 1854, cinnamaldehyde was synthesized in a laboratory for the very first time by an Italian chemist, Luigi Chiozza.

Because I would love to explore chemistry, let’s talk about cinnamic aldehyde structure, synthesis, and more.
Structure of Cinnamic aldehyde
Generally, having molecular formula C9 H8 O, if we talk about functional groups in cinnamic aldehyde, it primarily represents compounds having two unsaturated groups, i.e., A C-C double bond and an aldehydic group. The cinnamaldehyde, is both an aldehyde and aromatic, has a benzene ring attached to an unsaturated aldehydic group situated primarily in the trans position. That is why most naturally occurring cinnamaldehyde is in trans (E) isomers.
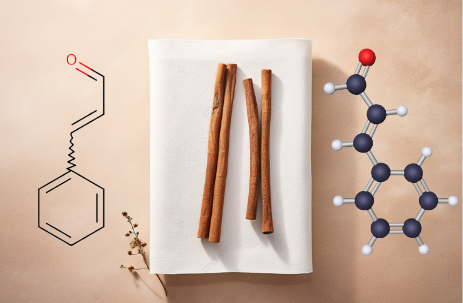
Based on the structure shown in the image, the IUPAC name of this compound is (E)-3-phenyl-2-propenal.
Cinnamaldehyde Properties
Molar mass: Having molecular formula C9 H8 O, cinnamaldehyde molar mass is 132.16 g/mol.
Density: Cinnamaldehyde density is 1.05 g/mL at 25 °C.
Melting and boiling point: The cinnamaldehyde boiling and melting point are −9-−4 °C and 248 °C, respectively.
Appearance: The question may arise if cinnamic aldehyde is solid, liquid, or gas. Usually, cinnamaldehyde is a yellowish to greenish-yellow liquid.
Viscosity: Cinnamaldehyde is more viscose than water, having a value of 21.25mm2/s.
Odor and taste: Cinnamaldehyde smells like cinnamon, has a strong, spicy aroma, and has a burning taste.
Solubility: If you are concerned about cinnamic aldehyde solubility or while reading its properties, you may think for once, can cinnamon dissolve in water? So the answer is usually cinnamaldehyde, which is insoluble in water and many organic solvents except for alcohols and some flavoring oils, which are pretty miscible.
Stability: Cinnamaldehyde is stable. But what does it mean? Do you think that cinnamic acid is flammable? Then, it is a combustible liquid that may result in fire or explosion when exposed to higher temperatures. So, if you have one with you, beware and keep the temperature below 30C. Don’t forget to avoid keeping it away from open flames and other potential ignition sources as per the product safety assessment.
Vapor density: The vapors of cinnamic aldehyde are heavier than air, having a density of 4.6. They can readily oxidize in air to form toxic substances and sometimes decompose into styrene.
Though getting bored of discussing that much chemistry of cinnamaldehyde, let’s talk about what cinnamic aldehyde is found in? or what foods contain cinnamaldehyde. Interestingly, it is present in various foods, households, and perfumes. (If you want to explore more about perfumes, jump to Ketone perfume). Cinnamaldehyde is in different foods, like cinnamon leaves, cinnamon, celery seeds, cassia leaves, tomatoes, lemon balm, and clove stems. If you like eating chocolate, you have already encountered cinnamaldehyde. The rarest amount of cinnamic aldehyde is also present in orange citrus.
Cinnamic aldehyde Synthesis
Cinnamic aldehyde results from biosynthesis, which begins when non-oxidative deamination of L-phenylalanine takes place with the aid of phenylalanine ammonia lipase (PAL), which gives rise to trans isomer of cinnamic acid.
Moreover, this trans-cinnamic acid converts to cinnamoyl-CoA with 4-coumarate–CoA ligase (4CL). Later, cinnamoyl-CoA reductase (CCR) reduces cinnamoyl-CoA to form cinnamic aldehyde.
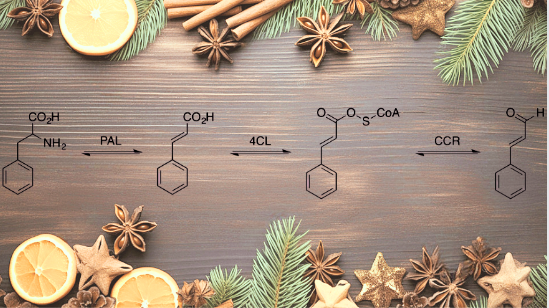
Apart from biosynthesis, cinnamic aldehyde has been synthesized in laboratories. One of the budget-friendly approaches is the steam distillation of essential oil from the bark of cinnamon. Furthermore, if we talk about non-natural synthesis sources, the listed are acetaldehyde and benzaldehyde, which form the product in a famous cinnamaldehyde aldol condensation reaction.
Derivatives of Cinnamic aldehyde:
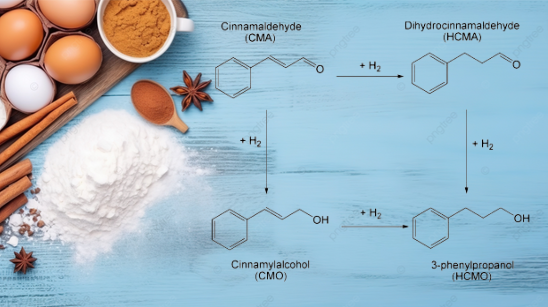
The selective hydrogenation of cinnamaldehyde results in various derivatives depending on the pathway followed, like Dihydrocinnamaldehyde, 3-phenylpropanol, cinnamyl alcohol, etc [1]. Other derivatives of cinnamaldehyde include hexyl cinnamic aldehyde, α-Amylcinnamaldehyde, alpha-methyl cinnamic aldehyde, and amyl cinnamic aldehyde used in fragrances or creating scents.
Cinnamic aldehyde uses:
Flavoring agents: The most apparent application of cinnamaldehyde is found in foods and medicines to enhance their quality in aroma and taste. It is widely used as a flavoring agent in chewing gums, ice creams, beverages, and candies to give artificial cinnamon flavor.
Perfumery: It is widely used in perfumes to recreate the ambiance of fruity, natural, earthy, and pleasant scents. Dihydrocinnamoyl alcohol, a cinnamaldehyde derivative, has a lilac and hyacinth fragrance. Similarly, cinnamoyl alcohol has an odor of lilac. Moreover, alpha-amyl cinnamaldehyde is a vital ingredient in the fragrance industry.
Therapeutic agents: There is a wide-ranging classification of therapeutic uses of cinnamaldehyde, such as Trans-Cinnamaldehyde, which possesses substantial anti-microbial activity[2]. The cinnamaldehyde can improve glucose homeostasis in diabetes. Hence, it has hypoglycemic effects[3].
Apart from this, cinnamaldehyde also has anti-microbial properties. Cinnamaldehyde has been reported to inhibit filamentous molds, yeast, and bacteria through cell wall biosynthesis, cell membrane alteration, or ATPase inhibition. Trans-cinnamic acid prevents about 50% of bacterial growth in oral cavities.
Furthermore, this fantastic chemical compound has been recognized for its apoptotic activities against various cancer cells and for possessing anti-HIV potential.
That is enough for now; knowing cinnamic aldehyde is good, but it is one thing that sparks your knowledge. Do you know what poison tastes like cinnamon? The answer is coumarin, a chemical compound used for rat poisoning. It can cause liver and kidney damage in humans, even if taken in minimal amounts.

This content is comprehensive and provides valuable insights into cinnamic aldehyde. I appreciate how the website presents the information in a clear and concise manner, making it accessible to both experts and beginners. Overall, it’s a fantastic resource for anyone seeking knowledge about cinnamic aldehyde. Keep up the great work, wisechemist.com! 👍
[…] flavour for every chocolate lover. Each bar is infused with honey and almond nougat and cinnamic aldehyde. This will adding a delightful crunch and depth of flavour that sets Toblerone apart from other […]