Unlocking the Power of Chemical Bonds: Exploring Types of Bonds and Lewis Structures with the Lewis Model
when it comes to the understanding chemical and physical properties of elements, the outermost shell electron becomes the primary concern as these electrons are involved in forming the chemical bond and in chemical reactions
Introduction to the Lewis Model
In 1916, an American chemist Gilbert N. Lewis presented a model that simplified many other observations about chemical bond formation and the way elements react. Lewis observed high degree stability of electron configurations of inert gasses i.e. Helium with a valance shell of two electrons (1s2) following the duplet rule (in which the outermost shell has the complete set of electrons), Neon (2s2 2p6), and Argon (3s2 3p6) with a valance shell of eight electrons respectively.
Why do atoms bond
Lewis explains that every other atom except nobel gasses tends to achieve an outer shell of eight valance electrons and is particularly common among main group elements (Group 1A-8A). and is given the name octet rule.
Understanding Lewis Structures
To show the outermost electrons or valance electrons of an atom, Lewis devised a notation where the atomic symbol represents the “Core” (i.e. the nucleus and all inner shells). This atomic symbol is neighbouring by the number of “Dots” that show the number of valance electrons in the outermost shell of an atom of that element. Such a representation is known as Lewis structures or Lewis dot structures.
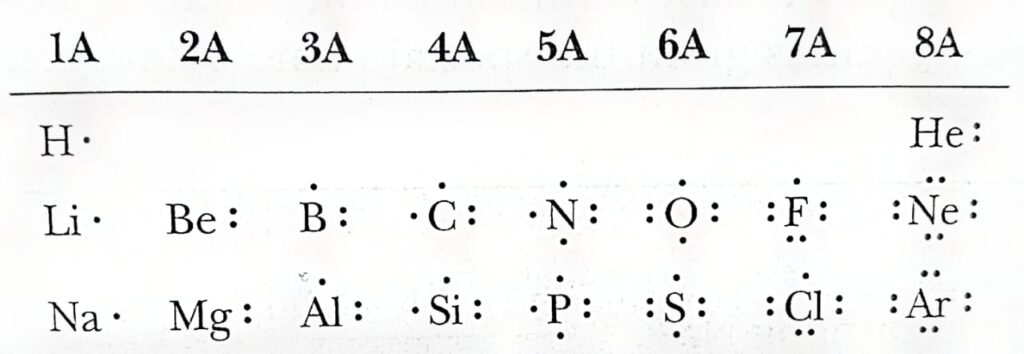
The Nobel gasses helium, neon, and argon have complete valance shells such that the valance shell of helium is fill with two electrons and that of neon and argon is fill with eight electrons for neon and argon the s and p orbitals are fill with eight electrons therefore they have common electron configuration. All other elements contain less than eight electrons in their valance shell see the image below.
Lewis Model & Formation of Chemical Bonds
According to Lewis’s theory, to attain stability atoms bond together in such a way that each participating atom tries to acquire a completed valance shell electron configuration similar to that of the nearest noble gas to it in its atomic number. The two common ways by which atoms acquire complete valance shells are:
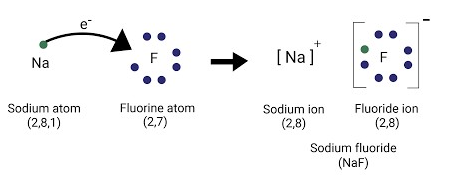
- Ionic Bond: To attain a filled valance shell an atom may lose or gain enough electrons hence forming ions. An atom that loses electrons becomes positively charge and is call a cation and an atom that gains electrons becomes negatively charge known as an anion. The electrostatic force of attraction between cation and anion results in the formation of an ionic bond. During the formation of an ionic bond the electrons transfer from the valance shell of an atom having lower electronegativity (loses electron to form cation) to the atom having higher electronegativity (gains electron to form anion). For an ionic bond to be form, the electronegativity difference between the two bonded atoms must be equal to 1.9 or greater. An example of an ionic bond formed between Sodium (having electronegativity 0.9) and Fluorine (having electronegativity 4.0). The arrow shows the transfer of one electron from sodium to fluorine, see image below.
- A covalent bond is a chemical bond that involves the sharing of electrons between two or more atoms. It is setup when two atoms share one or more pairs of electrons to achieve a stable electron configuration. This type of bonding is typically occur in nonmetals and is characterize by its strong, directional nature. When two hydrogen atoms make a covalent bond, the single electron from each atom contributes to make a bond and hence the shared pair completes the valance shell of each atom. More examples includes the type of bonding in ethers, ketone, phenols etc.
Lewis Model & Bond Length
Lewis model describes that during the formation of a covalent bond between two atoms, the region between the two nuclei of atoms is occupied by an electron pair. Cosequently, it hinders the repulsive forces of the two positively charged nuclei. Similarly, the pair of electrons in the space between the two atoms attract the nucleus of atoms. This fixes the internuclear distance to a very narrow range. Therefore, the distance between the participating nuclei is called a bond length in a chemical bond.
Lewis Model & Structures of Polyatomic Ions
We need to consider the following steps while applying Lewis theory for molecules and polyatomic ions.
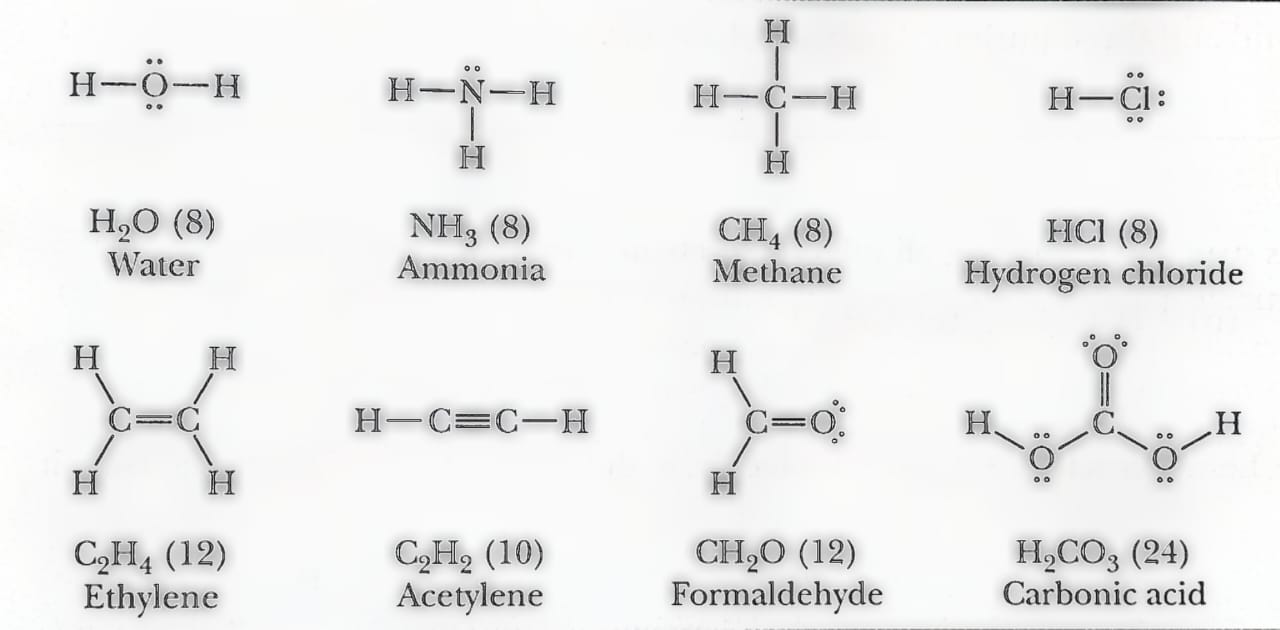
- Identify the number of valance electrons contributed by each atom in the ion (by adding one electron for each negative charge and subtracting for each positive charge on the ion) or molecule. For instance, the Lewis structure of water (H2O) must have eight electrons. Two of them are contributed by the hydrogen atoms and six from oxygen
- To identify the connectivity of atoms in the ion or molecule, Lewis structure advises connecting atoms with a single bond and then positioning the lasting electrons in pairs around the atom to show a complete octet. For example, each atom of hydrogen atom must be surrounded by two electrons. Similarly that of carbon, nitrogen, and oxygen by eight electrons as per the octet rule.
- Bonding electrons comprise a pair of electrons that participate in covalent bonds andare shown by a single line between atoms. Whereas nonbonding electrons are unshared pairs of electrons displayed by dots around the atom.
- In a single covalent bond, two atoms share a single pair of electrons (i.e. one electron from each atom). In a double covalent bond, the bonded atoms share two pairs of electrons. While in a triple covalent bond, the bonded atoms share three pairs respectively. Lewis structures for various compounds are shown below.
Formal Charge
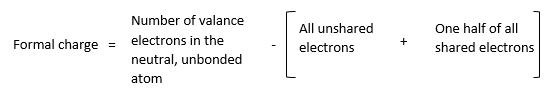
The charge that appears on an atom in a polyatomic ion or molecule is called a formal charge. Based on charge appearance, polyatomic ions are classified as polyatomic cations (examples include hydronium ion H3O+, and ammonium ion NH3+) and polyatomic anions (example is the bicarbonate ion HCO3+). Lewis theory gives the following steps to derive a formal charge.
- Draw an accurate Lewis structure for the molecule or ion.
- Designate all the nonbonding (unshared) and bonding (shared) electrons to an atom.
- Compare this value with the number of valance electrons in the unbounded neutral atom. Suppose the number of electrons assigned to a bonded atom is greater than that assigned to an unbonded neutral atom. In that case, there are more negative charges in the nucleus. Thus, the atom has the negative formal charge and vice versa.
[…] If this information piques your interest, be sure to explore more about intriguing mysteries in chemistry by knowing about lewis model of bonding […]