Whether discussing mesmerizing art created with alcohol markers or the secrets of scents revealed by ketone perfumes, it is high time to delve into the captivating world of phenolic crystals. These crystals’ stunning and aesthetic symmetry attracted scientists a long time ago. But before we indulge in the chemistry of phenol crystals, let’s first look at what phenol is.
Understanding phenol.
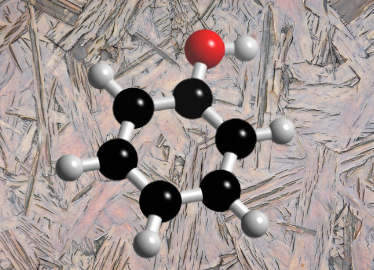
Phenol , a cyclic modification of alcohol, belongs to a class of organic compounds in which the (-OH) group is attached to a C-atom and directly connected to an aromatic ring (the General structure is shown below). Phenol is also well known as carbolic acid or benzol. Mainly, phenol is a generic name assigned to the most common member of the family, monohydroxybenzene (C6H5OH).
If we talk about the general properties of this compound, phenol is a volatile, white crystalline solid. We got to know that there is a significant difference between phenol and its counterpart alcohol. The alcohol has the (-OH) group is bound to a saturated carbon. Meanwhile, the (-OH) group in phenol is bound to unsaturated carbon, which stabilizes due to resonance. Consequently, phenols have higher acidity than alcohols for the very same reason.
What are Phenol Crystals?

Famous for its medicinal and sweet odor, phenol crystals are white crystalline solids that sometimes appear pale yellow or pink. Usually formed by the crystallization of phenol, these crystals have a symmetrical appearance, which is majorly due to the intermolecular interactions between phenols.
Formation of Phenol Crystals:
The simplest yet extraordinary structure of phenol interacts in such a diverse form that it can create a wide range of crystalline phenol with symmetry elements like screw axes, inversion centers, and glide planes, etc. some of such intermolecular attractions are:
- The parallel phenol-phenol stacking interaction forms hydrogen bonding between C=O and –C-H groups.
- Antiparallel phenol-phenol interaction forms hydrogen bonds with surrounding molecules.
- Antiparallel interactions result in hydrogen bonds between pie-bonds of –CH.
- Antiparallel phenol/phenol interaction with the parallel −OH groups.
- Parallel phenol/phenol with interaction of the parallel −OH groups.
These are the few intermolecular attractions phenol undergoes to give rise to visually stunning crystalline morphologies with remarkable symmetries.
Characteristics of Phenol Crystals:
Physical Characters:
1 Appearance: Crystalline phenol is usually colorless to white or sometimes pale pink or yellow crystals, possessing remarkable transparency.
2 Luster: Phenol crystals exhibit a lustrous, bright presence, which makes them visually enticing.
3. Solubility: Here is the answer if you wonder how to dissolve phenol crystals. Because they evaporate slower than water, Crystalline phenols are slightly soluble in water. These crystals are also highly soluble in organic solvents like alcohols, acetone, ether, and benzene, which makes them useful for many organic syntheses.
4. Melting and boiling point: Phenol crystals’ melting and boiling points range from 40 – 42°C to 181 – 182°C.
Chemical characters:
- Molecular weight The molecular weight of phenol crystal is 94.11.
- Reactivity: Crystalline phenol becomes highly reactive when exposed to direct sunlight or high temperature.
- Stability: Phenol crystals are generally stable when separated from compatible materials like halogens, alkalis, and strong oxidizing agents. They remain stable in an airtight container, well-ventilated, and at a cool temperature.
Toxicity of Phenol Crystals:
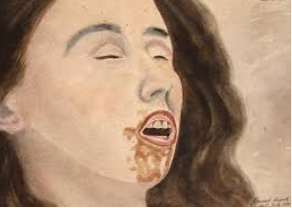
- Skin contact: phenol crystals, when they come in skin contact, can cause severe skin burns, including blistering of skin along with pain, itching, and redness.
- Inhalation: overexposure to crystalline phenol proves corrosive to the respiratory tract, including nose and throat irritation.
- Eye contact: A single exposure can lead to serious eye damage, causing pain, abundant watering of the eyes, and redness.
- Ingestion: Accidental intake of phenol crystals causes severe mouth burns to lead to esophageal and stomach damage followed by pain, nausea, and vomiting.
Preventive Measures:
Crystalline phenol tends to be very toxic and corrosive; therefore, careful handling is foremost in practicing these crystals. You may take the following measures if you encounter any of the exposures mentioned earlier:
If skin contact occurs, wash off the product immediately by rinsing the affected area with water for at least 15 minutes. Prefer medical advice for chemical burns. In cases of accidental ingestion, administer rapid treatment by thoroughly washing the mouth with water and then drinking plenty of milk unless seeking medical attention.
If you accidentally inhale these crystals, try to move out in fresh air and exhale deeply to overcome the side effects.
Before working with these crystals, one should consider careful handling and follow properly equipped protocols.
Uses of Phenol Crystals:
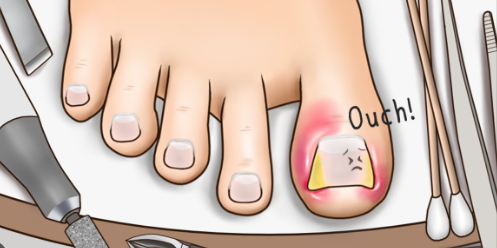
- Medicinal uses: Doctors widely use crystalline phenol to treat various skin diseases. Podiatrists and chiropodists use penalization to treat nail avulsion (losing fingernail or toenail due to injury) or perform matrixectomy (removal of ingrown curved toenail). Furthermore, phenol crystals prevent nail regrowth when smeared on the nail bed.
- Chemical synthesis: Crystalline phenols are invaluable in the synthetic industry. They are primarily used to form phenolic resins and synthetic fiber and can also be employed in the pharmaceutical and dye industries.
- Molecular studies: Researchers and education specialists widely employ crystalline phenols in X-ray crystallographic studies to understand crystals’ diverse molecular structures and intermolecular attractions.
Interestingly, you may wonder how to make a phenol solution from phenol crystals. Here is the tip:
- Take specific grams of phenol crystals (for instance, 500 gm).
- Put them in 500 ml 50 mM Tris-Cl pH 8.
- Shake well until dissolved.
Don’t forget to use a fume hood.

[…] answer is simple. Usually, phenols undergo Williamson etherification similar to alcohols. Unlike the formation of alkoxide (the […]
[…] the valance shell of each atom. More examples includes the type of bonding in ethers, ketone, phenols […]
[…] interaction may also occur with organic solvents like (alcoho, phenol […]
[…] and rapid drying of ink. Likely, such compounds provide fluidity (chemistry says alcohols and phenols have low […]